Structural Sensitivities in Bimetallic Catalysts for Electrochemical CO2 Reduction Revealed by Ag−Cu Nanodimers
Posting date on solidfuturism: February 29th 2024
Published date: Jan 18th 2019
Authors: Emad Oveisi, Jianfeng Huang, Mounir Mensi, Valeria Mantella, and Raffaella Buonsanti
DOI: https://dx.doi.org/10.1021/jacs.8b12381
Abstract composer: Seyed Amirhosein Mirsadri
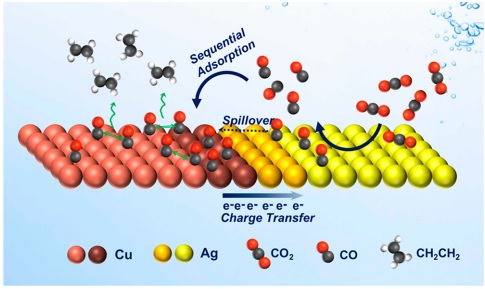
Considering the importance of carbon capture technology and the conversion of carbon dioxide gas as one of the main greenhouse gases that can have potentially bad effects on nature and the life of living beings, many scientists are paying more and more attention and research on this branch. When we can convert a pollutant gas into better fuel and non-fuel compounds with higher added value and at the same time reduce air pollution, why not do it? In the past, it was pointed out that copper metal can be a very suitable electrocatalyst for creating compounds such as methane and carbon compounds with more than two carbons. But as many of these electro-synthesis studies have been more focused on alloy nanoparticles or bimetallics, therefore the importance and impact of the shape and structure of metal nanoparticles on the efficiency and selectivity of the carbon dioxide reduction reaction is more felt. For example, if copper is combined with gold, carbon monoxide, if copper is combined with palladium, methane and if copper is combined with zinc metal, we reach ethanol. Also, the combination of silver with copper can have a significant impact on the reactivity and selectivity of copper metal, therefore in this article, the effect of this metal has been tried to be studied, but not synthesized as an alloy!
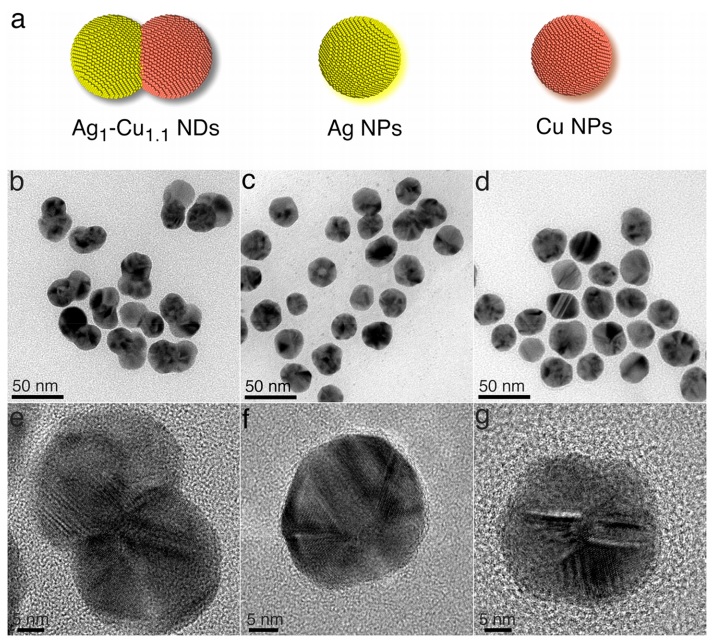
In fact, in this article, instead of the nanoparticles being completely atom by atom next to each other and completely combined and alloyed, two completely separate nanounits of copper and silver with a size of 25-30 nanometers are completely placed next to each other and form a metal dimer. The synthesis method of this type of special nanometer structures was by core growth, which was the primary core of 25 nanometer silver nanoparticles, and copper nanoparticles were grown next to it. Since the size and shape of nanoparticles are of great importance in the selectivity of the carbon dioxide reaction path, placing silver as a producer of carbonyl (precursor of multi-carbon compounds) next to copper, which is the most selective metal for multi-carbon and methane hydrocarbons, can be very beneficial. Fortunately, in the core-growth synthesis method, by changing the environmental conditions such as reducing agent and temperature, different sizes and shapes can be achieved. For example, the use of ascorbic acid in this synthesis was done to control the size and the high temperature of 150 degrees Celsius did not have much effect on it and since it caused the destruction of the synthesis vial cover; In this research, ascorbic acid by creating copper oxide causes the creation of large particles and sodium ascorbate causes very fast nucleation, which itself creates small nanometer particles, which can be a good control for the researcher to select and design the size of particles.
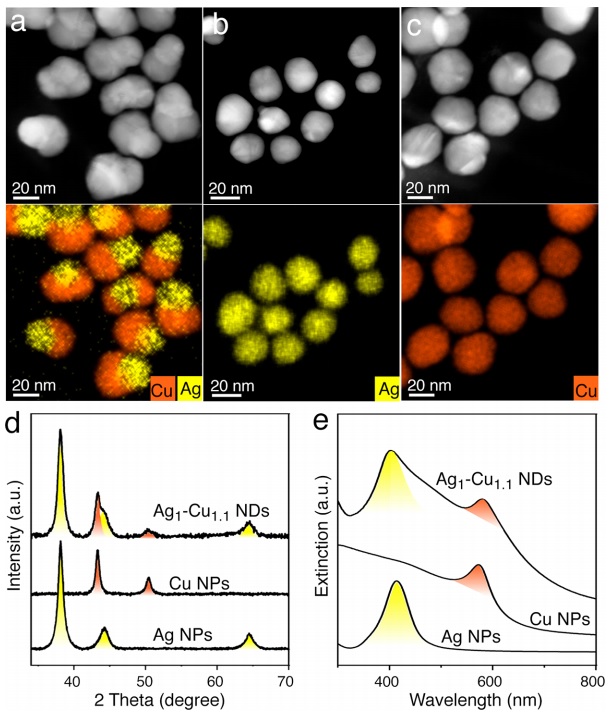
In previous published articles, the use of one-piece synthesis (One pot) that creates nanoparticles above 100 nanometers and core-shell synthesis of copper-silver and also the use of copper chloride precursor are more seen. But the chloride ion in the presence of silver and oxygen and high temperature can cause the erosion of silver and gradually reduce the size of silver, which is not seen in this article in the design of nanoparticle size. Considering that very good results were obtained in the production of electro-synthesis of ethene selectively and with very high efficiency in silver-copper nanoparticles with a ratio of 1 to 1, this assumption arises that whether increasing the silver metal (source of selective carbonyl) and reducing the size of copper metal or vice versa has a significant effect on the efficiency or not, which the answer was no. Rather, the ratio of the size of 1 to 1 metals had the most impact on the conversion of carbon dioxide to ethene. By creating an experiment, the result of mixing different percentages of nanoparticles separately in different ratios, this hypothesis was tested, which still showed a lower yield than the dimer 1 to 1.
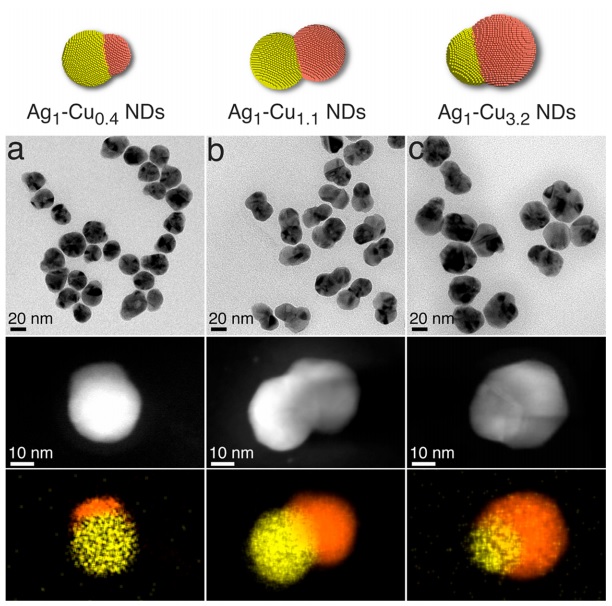
At the same time, silver-copper nanoparticles with a ratio of 1 to 0.4 and 1 to 0.23 also showed the same low yield as 1 to 1. Therefore, the hypothesis that we increase silver to carbonyl for the copper metal next to it to create more ethene or vice versa is not completely rejected, but to a very large extent it is not the main factor of this reaction! And other factors are involved at the nanometer level. This means that this reaction reaches the highest yield when the size and ratio of the two metal particles are in balance and balance can be the most influential parameter in the carbon dioxide reduction reaction. In this research, core-growth synthesis and also devices such as potentiometer with glass carbon electrode and XPS, HRTEM, XRD, FFT (Fast fourier transform), HAADF-STEM, UV-vis and EDX were used for identification and analysis.


klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)