Synthesis of new nanocomposite based on nanoceramic and mono substituted polyoxometalate, PMo11CdO@MnFe2O4, with superior catalytic activity for oxidative desulfurization of real fuel (This article)
Posting date on solidfuturism: January 18th 2024
Published date: May 27th 2020
Authors: Mohammad Ali Rezvani, Majid Hadi, Seyed Amirhosein Mirsadri
DOI: https://doi.org/10.1002/aoc.5882
Abstract composer: Seyed Amirhosein Mirsadri
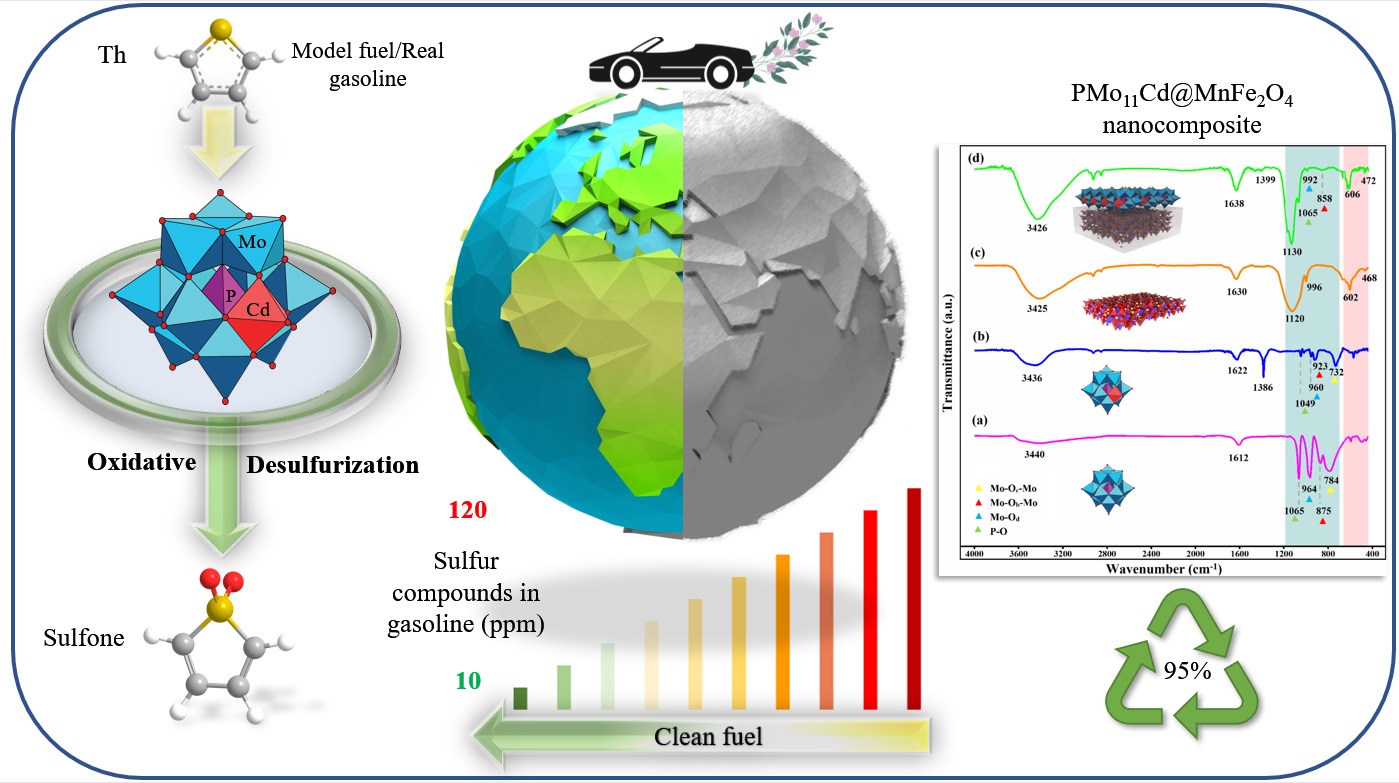
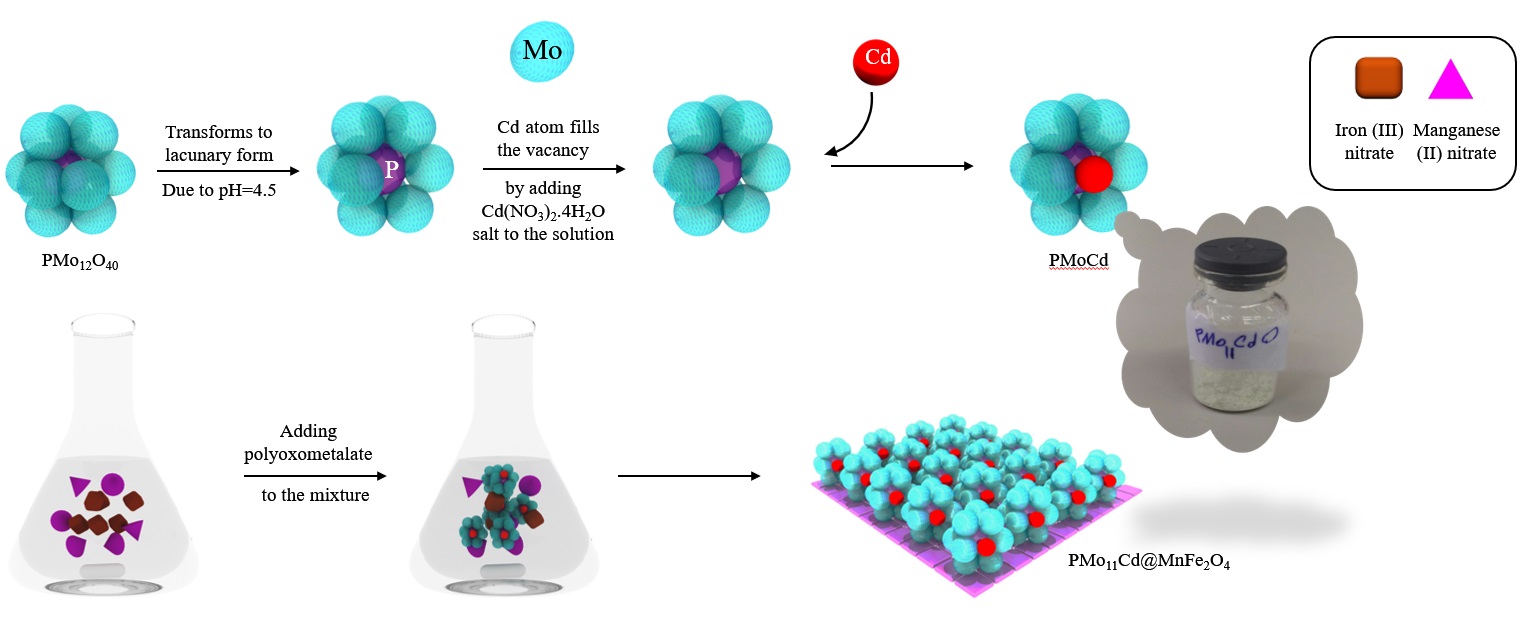
Polyoxometalates are kinds of inorganic polymers that has drawn much more attentions these days, as a matter of fact, just because of their huge amounts of negative charge cloud existant around their oxygens they have shown superior capabilities of reduction reactions. They can be formed through different medium pH, therefore, when it is more acidic it will form stable polyanion and when it is in basic medium it will decompose very orderly. in this oreder we can design lots of different and diverse known structures with vacancies or intact formation. To that end, by fluctuate the pH value, more addenda will be removed and we can decide whether to substitute new atom/molecule or not. The most famous keggin structure has a formation of XM12O40 that X (x=P, Si) is inner tetrahedra and m a central octahedra units which octahedras gathers together to make an encapsulation around the inner tetrahedra. M could be defined as mostly early transition metals. For more instance, keggin polyanion has 12 octahedras that surrounds the one inner tetrahedras. Therefore, the superior capabilities of the polyoxometalates make them good candidate for the oxidative catalytic pathways for cleaning the fuel from Di benzothiophene (DBT) , Benzothiophene (BT) and Thiophene (Th) which are the most sources of toxic SOx compounds. These toxic SOX compounds will be so distructive for environments so must be removed. By immobilizing this polyoxometalate on a firm substrate like MnFe2O4 the special surface to volume ratio will be increased and a new nanocomposite will show better abilities in the catalytic reactions.
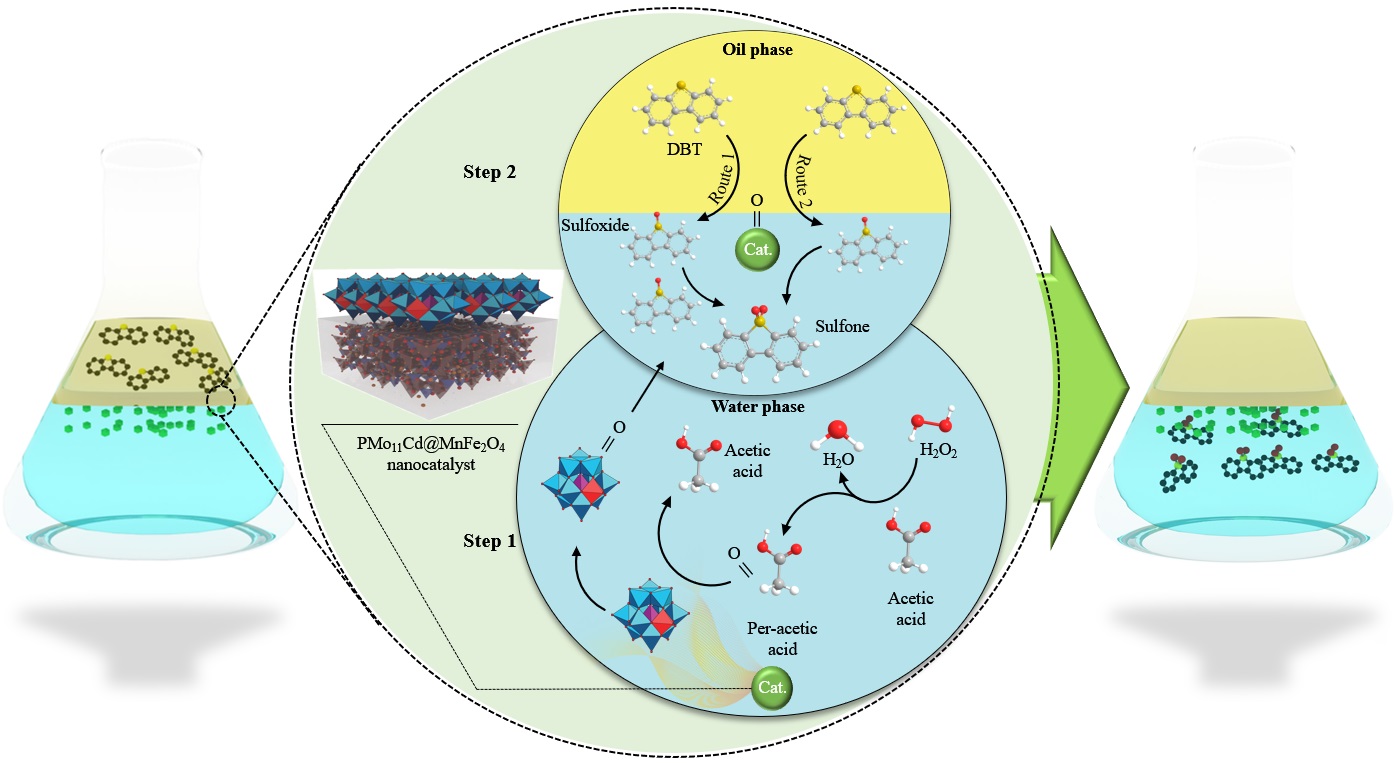
The former and dominant pathway for desulfurization was known as hydrodisulfurization which needed a much more energy with harsh conditions (still it is used in industries) to be applied which is not in our favour to do expensive conditions. The low cost catalytic pathways could be done by a proper catalysts like this as-prepared nanocompound PMo11CdO@MnFe2O4. The reaction performs in a heated flask in presence of a nanocomposite with a test fuel. The catalyst will be turned to a more reactive form of peroxo moieties by a proper solvent and then can be applied to the refractory sulfur compounds to turn them in a more polar form which can be then separated in a W/O two phasic reaction by a separation funnel.
The nanocomposite were characterized by a FT-IR, XRD, XRF, FESEM and EDX. The nanocomposite showed a good range of efficiencies around 95% before and after recyclability tests.


klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)