Efficient Methane Electrosynthesis Enabled by Tuning Local CO2 Availability
Posting date on solidfuturism: February 15th 2024
Published date: Jan 28th 2020
Authors: Xue Wang, Aoni Xu, Fengwang Li, Sung-Fu Hung, Dae-Hyun Nam, Christine M. Gabardo, Ziyun Wang, Yi Xu, Adnan Ozden, Armin Sedighian Rasouli, Alexander H. Ip, David Sinton, and Edward H. Sargent
DOI: https://dx.doi.org/10.1021/jacs.9b12445
Abstract composer: Seyed Amirhosein Mirsadri
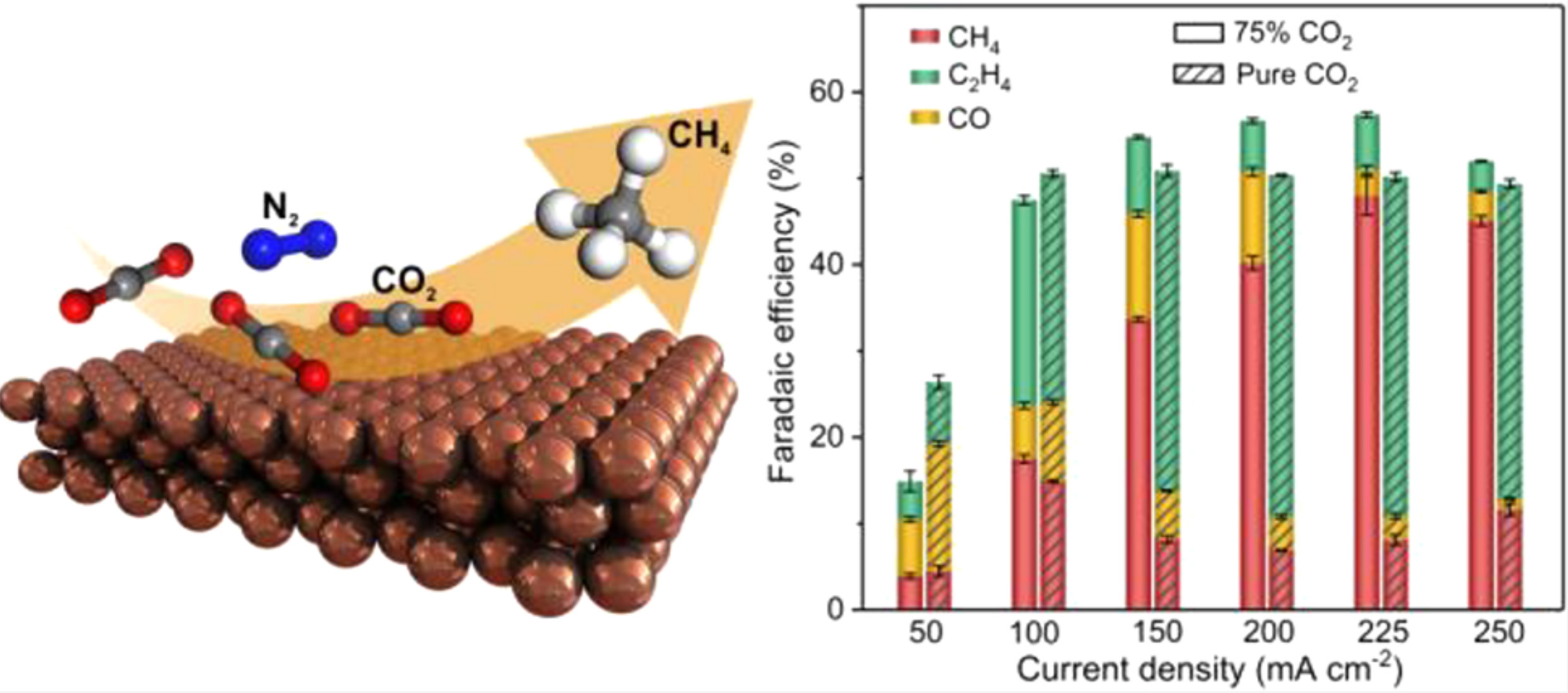
Can we convert carbon dioxide into other compounds by electro-synthesis and applying electricity by galvanostat in a constant current? The answer is yes. In an article that you will read now, a group of researchers from the University of Toronto found that by reducing carbon dioxide in an electrochemical cell in a constant current, they can obtain various products such as ethylene, ethanol, formate, carbon monoxide and methane. This article has tried to specifically follow the production of methane, from this perspective that in the past, various articles had been published on this subject, but the yield of methane was only 18% on average, which is a very small amount. For a long time, they have studied the effect of using copper metal in a selective way of the reaction towards methane, but increasing its yield in the electrochemical reduction system is important. Therefore, usually researchers have worked on the structure and size of copper nanoparticles, while this article tries to increase the yield of converting carbon dioxide to methane by changing the concentration of carbon dioxide absorbed on the cathode with controlling the applied current.
In this way, a flow cell was designed and a 1M solution of potassium hydrogen carbonate was saturated by a stream of carbon dioxide gas at speeds of 10 and 90 milliliters per minute. In the past, with a current density of 100 milliamps per square centimeter, they had only reached a yield of 18% and the reason was nothing but the preference of the reaction towards the carbon dioxide and dicarbon or tri-carbon molecules such as ethanol and etc. To investigate the reason for these results, the reactions after breaking the carbon dioxide molecule were examined, which were as follows: First, carbon dioxide is converted to the adsorbed intermediate(*) carboxylic acid* on the cathode, then converted to carbon monoxide* and in this part carbon monoxide* can either couple and reach higher carbons C2 and C3 or protonate and become aldehyde and then change to methane.
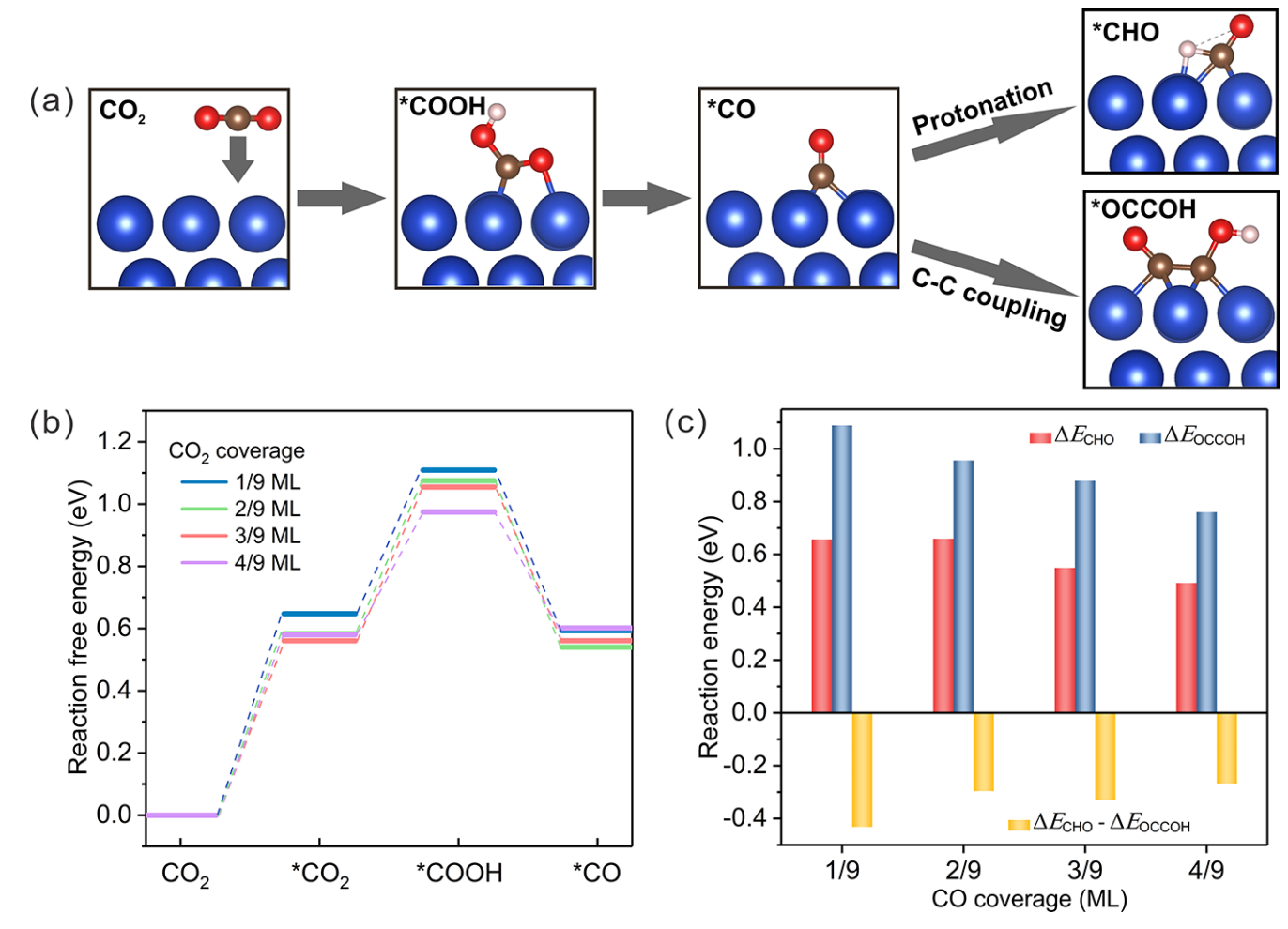
So in fact we prefer a reaction that is more protonated! Which itself can improve methane production. In this case, do we increase the concentration of CO2 to proceed the path we want? Actually the answer is no. In fact, Henry's partial pressure law proves that the concentration is directly related to the pressure of the applied gas and also the concentration is directly related to the absorption of gas on the electrode and its diffusion. Therefore, to predict the trend of this dispersion on the electrode, quantum density functional theory (DFT) simulations by the difference of free energy concluded that by increasing the concentration, the reaction loses its selectivity for methane! Therefore, several modes were designed to perform the reactions. Saturated carbon dioxide gas mode, 25, 50 and 75% of carbon dioxide to nitrogen gas ratio, which these gases before entering the solution first were mixed in a mixing chamber. The yield of 100% mode was very low, but at the same time they concluded that if we increase the applied current density, we will reach higher yields. So they tested lower percentages, which as the percentage of carbon dioxide composition decreases, the yield of converting it to methane specifically on the copper electrode increases! Therefore, they increased the current density and at the same time reduced the ratio to reach a remarkable yield of 48%, which was much better than the previous 18%.
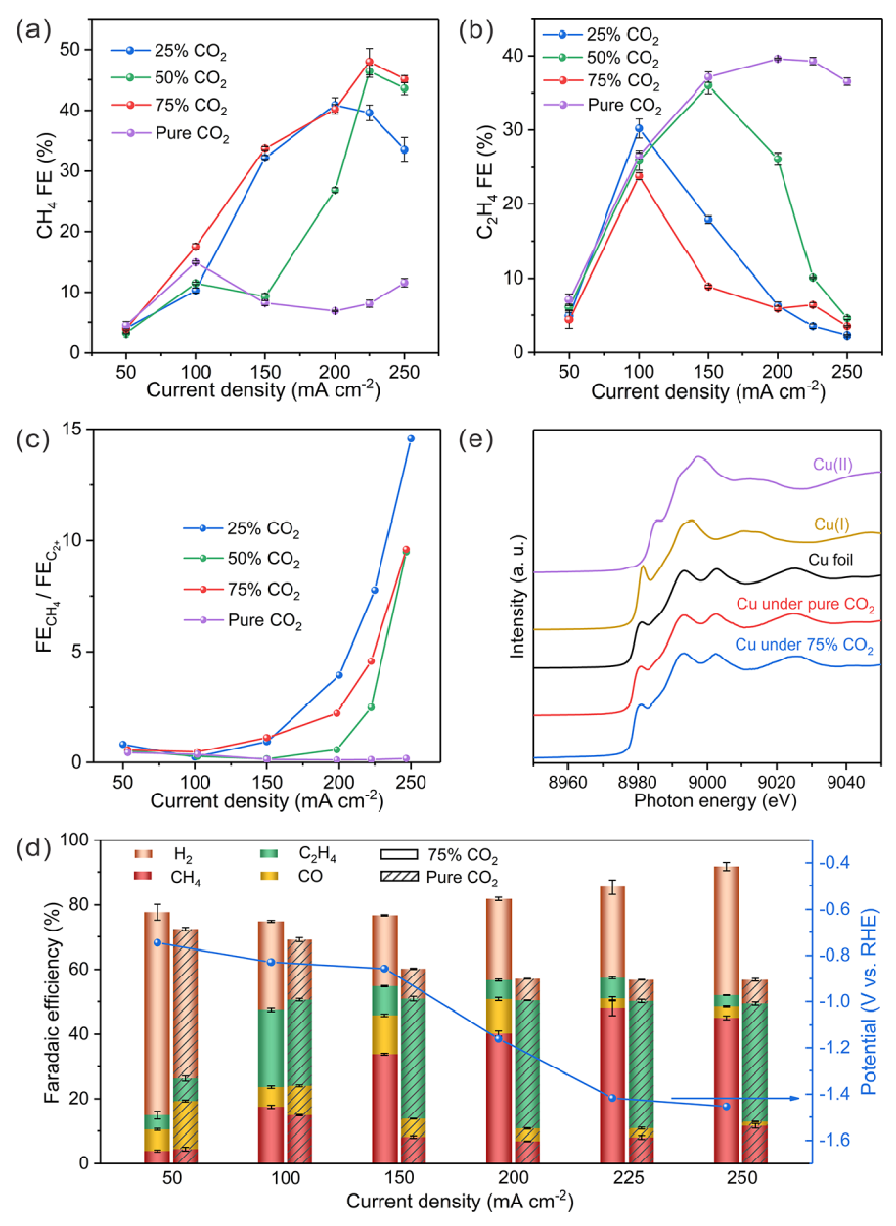
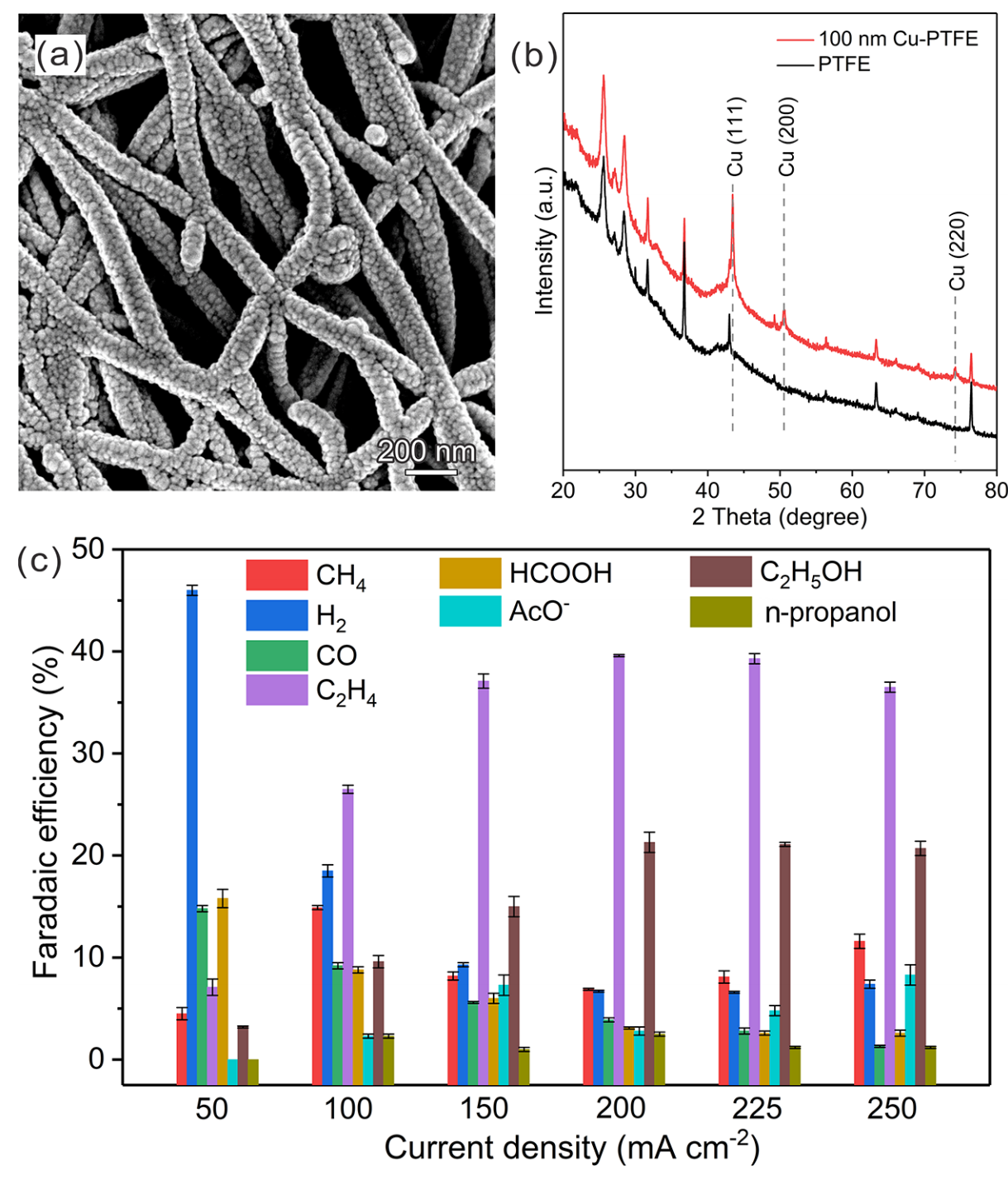
The optimal energy consumption value of 20% for converting this yield was obtained at a much cheaper way in the ratio of 75%. The reason why this yield is obtained is apparently simple and nothing but more absorption of hydrogen gas. Since in the lower ratio, the electrode surface can hold more hydronium ions and convert it to hydrogen, this hydrogen can enter a better reaction with the intermediate carbon monoxide in the next steps and take it more towards aldehyde and finally methane and therefore more yield is obtained. This experiment proved that the cell in the current and dynamic flow can perform well for 22h continuously, which only reduced its yield by 6% and reached 42% from 48%. In this experiment, a gas chromatography (GC) was used to evaluate the gas phase contaminants on the solutions and also to examine the solution and liquids of it, a nuclear magnetic resonance (NMR) device was used. Also, to detect the correct fabrication of copper metal on polytetrafluoroethylene polymer with a thickness of 100 nanometers, which was done by sputtering and evaporation, an X-ray diffraction device and also an electron microscope were used to confirm. The pure carbon dioxide gas flow was 10 milliliters per minute and the mixed gas flow was 90 milliliters per minute.


klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)