Tunable Cu Enrichment Enables Designer Syngas Electrosynthesis from CO2
Posting date on solidfuturism: February 22th 2024
Published date: June 29th 2017
Authors: Michael B. Ross, Cao Thang Dinh, Yifan Li, Dohyung Kim, Phil De Luna, Edward H. Sargent, and Peidong Yang
DOI: https://dx.doi.org/10.1021/jacs.7b04892
Abstract composer: Seyed Amirhosein Mirsadri
Nowadays, many scientists have directed their research towards carbon-based syntheses that usually start from the absorption of carbon dioxide gas. This means that we convert carbon dioxide into other good carbon compounds in a way that we can better store or use. This work can have a great impact on atmospheric processes and, in addition to creating clean air, enable access to a new branch of renewable energies. To achieve a process that is both cheap and in normal and calm synthetic conditions, i.e. at ambient temperature and pressure, we need a very precise study of the behavior of materials in synthetic conditions. The electrochemical reduction of carbon dioxide is one of the branches of carbon dioxide synthesis that has attracted much attention today due to its performance at ambient temperature and pressure.
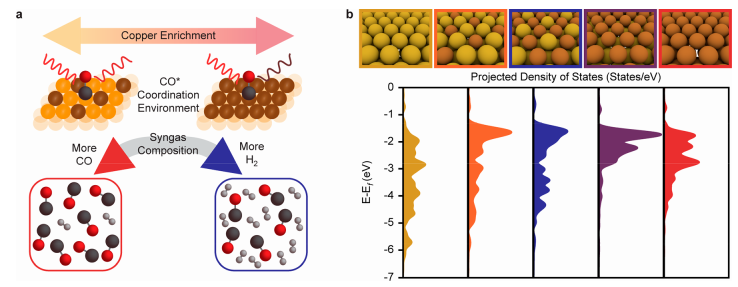
. In this article, an attempt has been made to change the structure of the gold electrode with copper to a structure for better conversion of carbon dioxide to carbon monoxide and also hydrogen. The gases synthesized from carbon dioxide are called synthetic gases or 'Syngas'. Since the electrochemical conversion of carbon dioxide can be converted into various carbon products, we need more selective processes and the study of this issue is one of the most important topics. Scientists have concluded that changing the gold electrode with copper in the electrochemical process can affect the conversion efficiency and selectivity of carbon monoxide and hydrogen resulting from the reduction of carbon dioxide. Not that we want to make an alloy of gold and copper metals, but we only get there by single-layer deposition by deposition and electrochemical underpotential deposition. In this method, we can deposit layers of less noble atoms such as copper on a more noble metal such as gold.
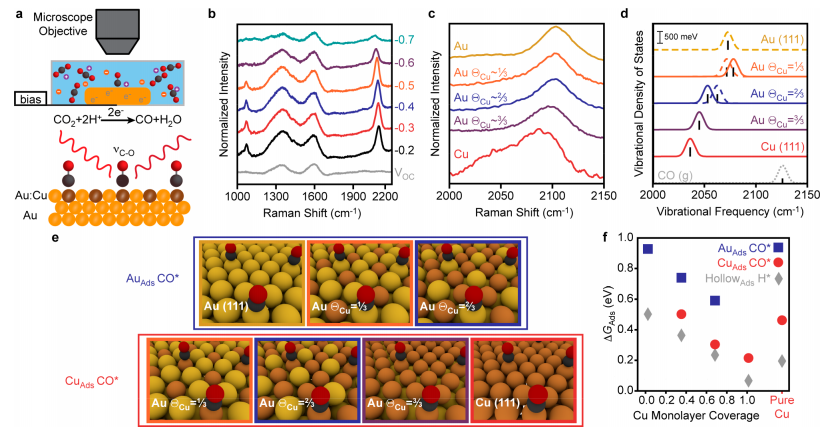
Also, this method can improve the Raman spectroscopy identification method for better tracking the production and creation of carbon monoxide and hydrogen. By controlling the synthetic conditions, layers with ratios of 1 to 3, 2 to 3 and 1 were obtained for the placement of copper atoms next to gold. In the meantime, the studies showed that carbon monoxide molecules can interact better with the orbital d of the copper metal from the oxygen head. Although carbon monoxide can bond with gold metal, the studies indicated that the deposition of copper atoms on gold can create better electronic coordination structures on the gold surface for better absorption of carbon monoxide and hydrogen molecules. This important information was obtained from the Raman spectrum study, which was taken directly from the electrode during the reaction, which was the result of diagrams that showed that when the molecular interaction between carbon monoxide and the orbital bond of the copper and gold atoms occurs, the red shift becomes more and stronger. All these studies were performed on the electrolysis of a half-molar solution of potassium hydrogen carbonate and all the results were confirmed by calculations (DFT).
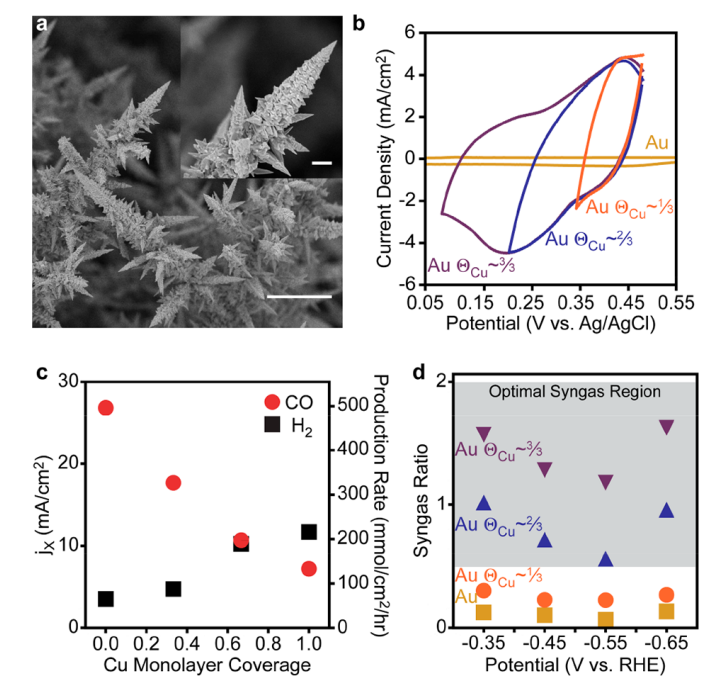
To better understand the absorption of carbon monoxide, the Gibbs free energy for surface absorption was studied, which showed that the absorption of hydrogen and carbon monoxide gas on the surface of the gold electrode compared to copper (1,1,1) is performed at high and positive energies, indicating non-spontaneity and reluctance. Also, all the tests indicated better absorption and desorption and reaction of carbon monoxide and hydrogen on the electrode surface. Therefore, the gold electrode covered by copper atoms with a ratio of 3 to 3 (i.e. a complete layer of copper atoms) showed a high yield of 70% for converting carbon dioxide to carbon monoxide. These reactions were investigated by Raman device, electron microscope, XPS and cyclic voltammetry.


klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)