Electrolyte design for lithium-ion batteries with a cobalt-free cathode and silicon oxide anode
Posting date on solidfuturism: February 8th 2024
Published date: OCT 19th 2023
Authors: Seongjae Ko, Xiao Han, Tatau Shimada, Norio Takenaka, Yuki Yamada & Atsuo Yamada
DOI: https://doi.org/10.1038/s41893-023-01237-y
Abstract composer: Seyed Amirhosein Mirsadri
We are all familiar with lithium-based batteries and even have some experience using them. These batteries can offer good capacity and power at a reasonable price, while some of them can be made from nature freindly materials. These batteries usually have an anode made of carbon or graphite, a cathode made of metal oxides that are alloyed with lithium, and an electrolyte made of organic materials that can retain lithium ions. However, many of these batteries use cobalt in their cathode structure. Unfortunately, cobalt is a rare element on Earth and is only found in Congo, where one of the problems of the mines is the use of child labor. In this article, instead of using a graphite anode, SiOx is used and in the cathode, instead of LiCoO2, LiNi0.5Mn1.5O4 is used. This compound is of interest due to its stability and higher potential than lithium, but the main issue is that the electrolyte that can be used for this system is not stable at high potentials and decomposes. In addition to this problem, another problem that arises for this system is the damage and corrosion of the electrodes at high potentials.
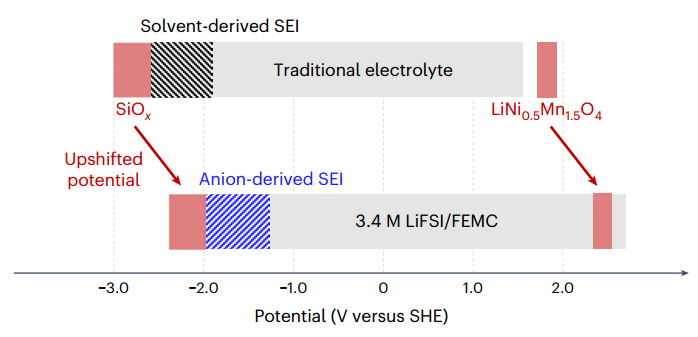
And these events are all dependent on the reactions and chemical potential of lithium with lithium ion that occurs in the electrolyte. To solve these problems and also maintain the potential and practical capacity of the 4.7 V battery described, we need to design a system with a suitable electrolyte that can maintain its stability in the maximum reduction range and not decompose, which also causes corrosion of the electrode (because the potential of converting lithium ion to lithium is out of the potential range of silicon oxide and lithium nickel manganese oxide). Therefore, additive electrolytes or electrolytes that create a passivation film on the electrode are used. For example, one can refer to electrolytes such as 1,2 dimethoxyethane tetrahydrofuran or those that contain fluorine such as fluoroethylene carbonate. To create resistance at high reduction potentials, one must be able to shift the electrolyte potential positively relative to the electrode, and this research group had concluded that the chemical potential of converting lithium to its ion, which was the cause of the positive potential shift, could be created by creating structures of connected ions (Li-Li, Li-ion) in macromolecular level. For this purpose, an electrolyte based on lithium nitrogen sulfur dioxide difluorinate/methyl (2,2,2 trifluoroethyl) carbonate was prepared.
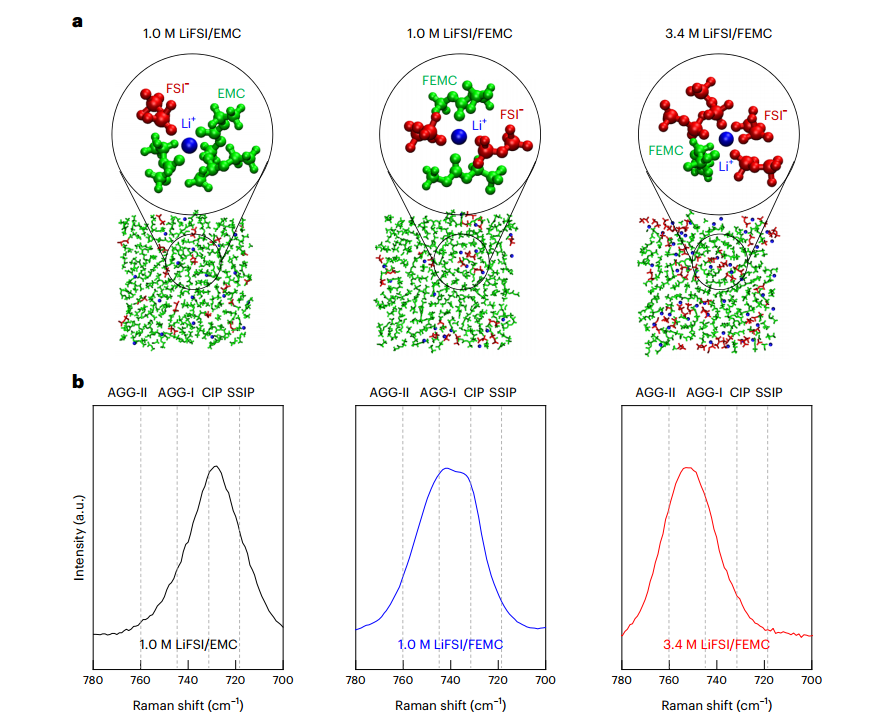
To understand the ability of creating ion-ion structure, computer simulation calculations were performed with Gaussian 16 software and the scientists realized that increasing the concentration could affect the creation of these structures, the highest concentration (3,4 M) was the optimal electrolyte state. To understand which electrolyte of the three different designed electrolytes could have reduction resistance, 80 cycles of slow charge discharge were used, which lasted three months with a constant current of 150 milliampers per gram, and the electrolyte 3,4 molar lithium nitrogen sulfur dioxide difluorinate/methyl (2,2,2 trifluoroethyl) carbonate showed the best capacity in percentage units than the others. By cyclic voltammetry experiments, it was determined that the reduction potential of the electrolyte shifted to higher potentials with increasing concentration and also the described electrolyte. Stability in oxidative cycles also remained constant due to the presence of electron-donating fluorine ions in electrolyte. Corrosion of metals such as aluminum occurs when aluminum penetrates the electrolyte as Al3+.
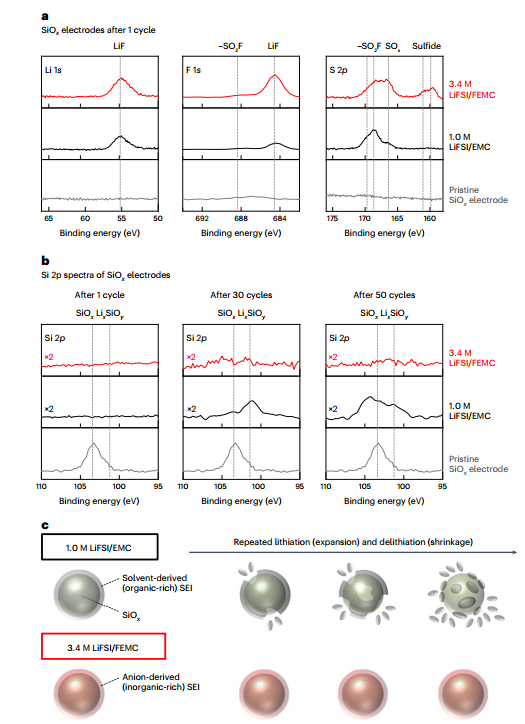
This happens less in this optimal electrolyte due to the fact that the solvaion of this electrolyte is much less and also the capacity to create ion pair structure in this electrolyte for elements such as aluminum and copper is very low (in this article silicon oxide and cathode were placed on copper and aluminum). All electrodes were made of lithium salt and the electrolyte was also commercially purchased and devices such as XPS, EDX, galvanostat and potentiostat were used for battery capacity, impedance tests. Also, this system was designed in a coin-like cell 2032 and cellulose, polypropylene and glass wool were used as separators.


klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)