A novel azo dye, 8-quinolinol-5-azoantipyrine as corrosion inhibitor for mild steel in acidic media
Posting date on solidfuturism: April 4th 2024
Published date: Dec 13 2007
Authors: Y. Abbouda, A.Abourrichea, T.Saffaja, M.Berradaa, M.Charroufa, A.Bennamaraa, H.Hannache
DOI: 10.1016/j.desal.2007.12.031
Abstract composer: Seyed Amirhosein Mirsadri
The corrosion of metals in the industry can have numerous destructive effects on industries and consequently on people's lives. From this perspective, scientists are looking for a solution to reduce metal corrosion. Many different metals and inhibitory materials have been studied in the presence of water and acid. For example, steel can be stainless, but to some extent, the alloyed iron atoms in steel can become somewhat corroded after a while in acidic solutions. To solve this problem, a group of researchers sought a way to synthesize color materials to prevent corrosion. Previously, mineral compounds such as nitrites, phosphonates, silicates, zinc, and cadmium, as well as organic compounds with nitrogen and oxygen atoms, had proven to some extent to cause inhibition in corrosion. Therefore, this path was investigated by researchers, and the organic azo dye 8-quinolin-5-azoantipyrine (HQAP) was used as a coating for the iron steel alloy. The reason for using this compound was that it would have less environmental impact compared to the previously mentioned molecules. These azo compounds are so suitable that they are even used in the pharmaceutical industry; also, their oxidation and reduction properties can make them suitable candidates for many chemical reactions.
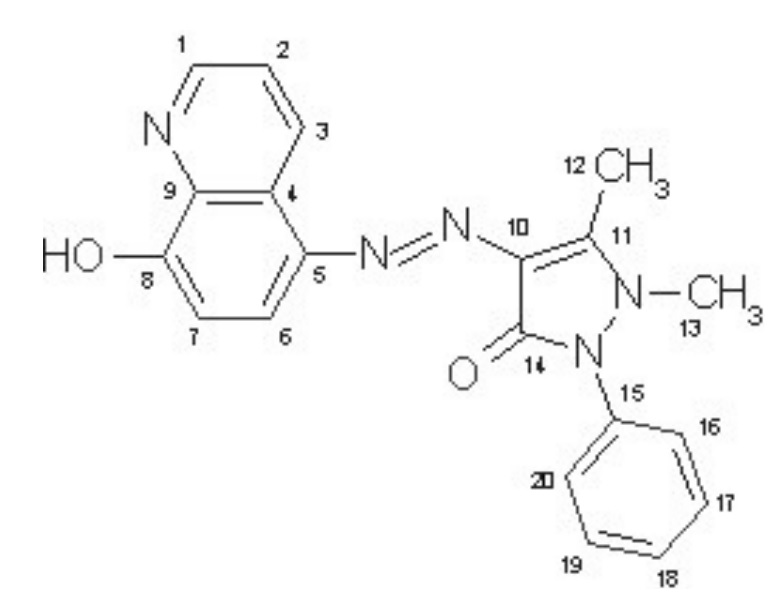
Therefore, a series of different experiments were conducted using very low concentrations of HQAP in a 1 molar hydrochloric acid solution. This series of experiments included weighing, spectroscopy, and potentiodynamic polarization evaluation (Tafel curve). In each of the experiments, the behavior of the iron ion and atom in the steel metal was examined. Since atomic dissolution in the solution can reduce the weight of the alloy, after several hours of the corrosion reaction, the changes in the weight of the working electrode were examined, and it was observed that in the absence of HQAP, corrosion increased, and in its presence, the weight of the working electrode decreased less. Similarly, the solution can be examined through UV-visible spectroscopy to determine how much iron metal enters the solution each hour. For this purpose, we can determine the amount of corrosion from the formula stated in the text and study the process yield further, which showed that at low temperatures and low concentrations, the yield increases up to 97%. These experiments showed that increasing temperature and concentration causes a decrease in inhibition and an increase in corrosion. These reasons were confirmed by the Arrhenius and Van't Hoff kinetic theories, which state that since this reaction is a type of physicochemical adsorption (an exothermic reaction that increases physicochemical adsorption), it is exothermic, and therefore its ΔG is negative, and the reaction is spontaneous.
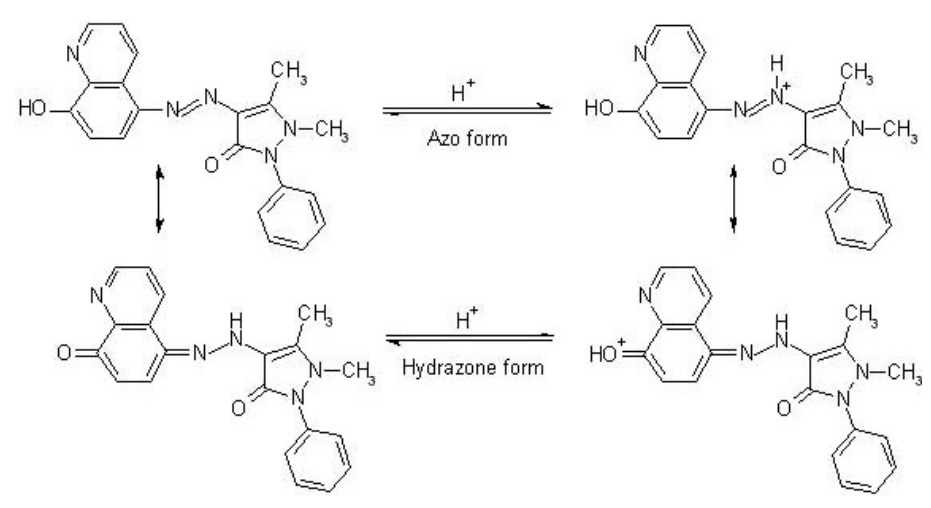
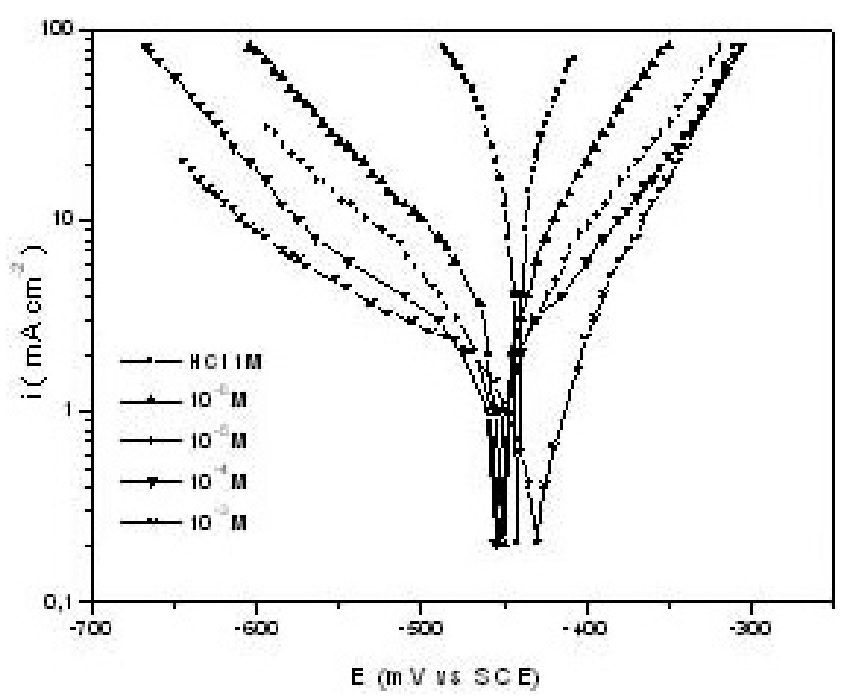
However, when the temperature rises, this Gibbs free energy for adsorption becomes more positive and can be absorbed less. The mechanism of this reaction is based on the fact that the azo compound can be adsorbed in two forms, isomer and tautomer, and thus by creating an iron complex with the HQAP ligand, it can physically prevent and inhibit both anodic and cathodic currents, or both. In contrast, the effect of concentration in the Arrhenius equation proved that the higher the concentration, the higher the activation energy of the reaction, which itself is a reason for making the reaction more difficult, leading to an increase in activation energy. Therefore, concentrations of 6-10 molar can be a good optimal amount. The Tafel curve of this reaction shows that the anti-corrosion substance HQAP has a dual feature, which can simultaneously affect both the dissolution of metal in the solution and the creation of hydrogen at the opposite electrode (meaning it can reduce both cathodic and anodic currents), with its cathodic effect being more significant. The parameter b for both anodic and cathodic currents showed a lower slope for anodic and a higher slope for cathodic, which makes us aware of the change in the cathodic reaction mechanism in the presence of HQAP, but the current and mechanism of the anodic reaction do not change. This category of colors can be a suitable option for coating steels used in the industry.


klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)