Tetrameric self-assembling of water-leansolvents enables carbamate anhydride-based CO2 capture chemistry
Posting date on solidfuturism: April 11th 2024
Published date: April 08 2024
Authors: Julien Leclaire, David J. Heldebrant, Katarzyna Grubel, Jean Septavaux, Marc Hennebelle, Eric Walter, Ying Chen, Jose Leobardo Bañuelos, Difan Zhang, Manh-Thuong Nguyen, Debmalya Ray, Sarah I. Allec, Deepika Malhotra, Wontae Joo & Jaelynne King
DOI: 10.1038/s41557-024-01495-z
Abstract composer: Seyed Amirhosein Mirsadri
Certainly, we need to devise solutions for controlling greenhouse gases. One of these gases is carbon dioxide (CO₂), primarily produced from hydrocarbon combustion. Currently, one of the most effective methods for capturing and converting this gas involves liquid-state absorbents. Ultimately, this process can yield methanol, ethanol, or methane. Among various aqueous and non-aqueous amine-based solutions, we can also utilize organic solvents and absorbents in non-aqueous environments. These alternatives offer both high absorption capacity and cost-effectiveness, with lower energy requirements. When considering blood circulation, oxygen easily moves through the bloodstream via hemoglobin. However, carbon dioxide cannot be transported as efficiently. Instead, it dissolves in the blood as bicarbonates and carbonate molecules. One such organic solvent and absorbent is normal 2-ethoxyethyl-3-morpholinopropyl-1-amine (abbreviated as EEMPA). EEMPA has good capabilities for capturing CO₂ by creating an intermediate without a proton, known as carbamic acid. In fact, this molecule can transform into polymeric or oligomeric forms in solution. These structures, facilitated by polar electronegative atoms and neighboring hydrogen bonds, can coexist alongside each other. In the fully capacitated state, four adjacent EEMPA molecules can effectively retain four CO₂ molecules, similar to how hemoglobin functions in human blood.

Studies using nuclear magnetic resonance (NMR) and density functional theory (DFT) modeling, based on thermodynamic data and activation energy constants, confirm that initiating the absorption of these compounds at very low concentrations and low pressures results in excellent absorption and the formation of better complete molecules. From this perspective, temperatures below 313 Kelvin and pressures below 15 bars are considered optimal reaction conditions. However, according to the obtained data, these oligomers form intramolecularly rather than extramolecularly, facilitated by the creation of intermediate states. "In this manner, the basic amino acid molecule, after absorbing carbon dioxide, transforms into a carbamate intermediate and then into protonated carbamic acid. During the initial loading, the molecule effectively operates at very low CO₂ concentrations to reach half of its capacity. Subsequently, higher capacities are created beyond saturation, meaning that initially, all molecules reach half capacity, and then this half capacity transitions to full.
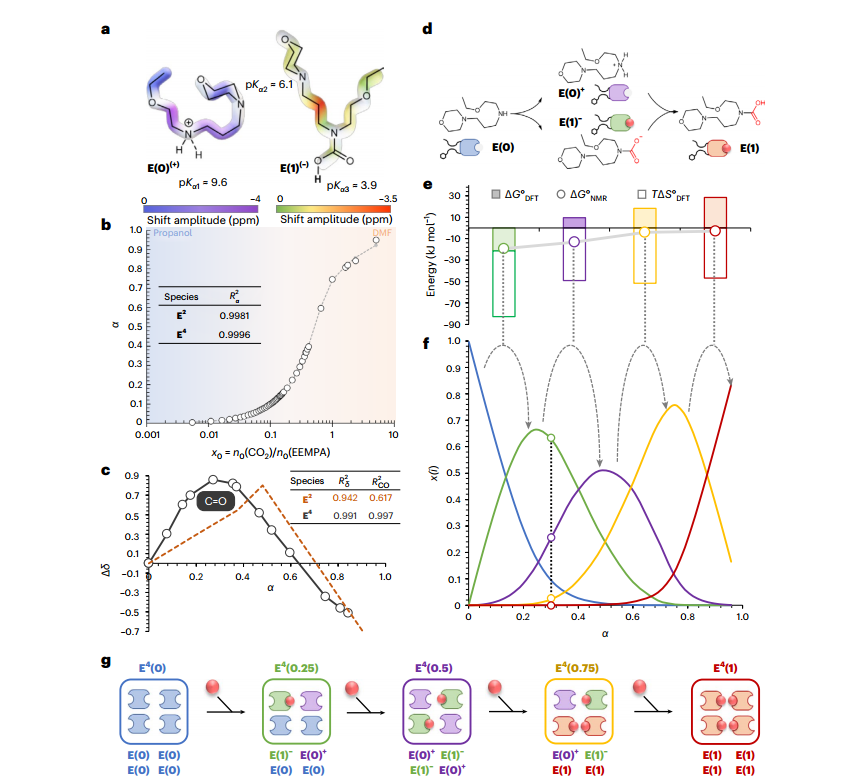
In this process, nuclear magnetic resonance (NMR) experiments have revealed that these compounds undergo structural changes during completion and saturation. Initially, they exhibit a planar shape, and at half capacity, they transform into a tetrahedral pyramid form. At approximately 75% capacity, they assume an approximate cubic shape, and at full capacity, they adopt an octahedral structure with eight faces, resembling ether-like structures at the corners of the octahedron. Additionally, it has been identified that the pathway for carbamic acid formation can occur through type II amines with two tail ends. Anhydride compounds suggest that in subsequent experiments, we can explore phosphate-containing compounds for carbon dioxide capture. Considering that these biomolecular compounds play various roles in the body, we can further investigate the use of adenosine triphosphate (ATP) or triphosphate adenosine for this purpose."



klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)