Hypoxia-triggered Nanoscale Metal-Organic Frameworks for Enhanced Anticancer Activity
Posting date on solidfuturism: March 14th 2024
Published date: jun 29th 2018
Authors: Ming Liu, Lei Wang, Xiaohua Zheng, Shi Liu, and Zhigang Xie
DOI: 10.1021/acsami.8b07570
Abstract composer: Seyed Amirhosein Mirsadri
Cancer is one of the common diseases these days in humans and animals. Many of the high-grade treatments for it are based on pharmaceutical chemistry and radiotherapy. Among these, radiotherapy has gained attention because it can have fewer adverse effects. If cancer progresses significantly and turns into a very hard and stiff dead tumor, it practically does not respond to radiotherapy or even chemotherapy and must be removed surgically. Therefore, for successful treatment, there is a need for living cells or tissues with oxygen. A cell loses oxygen when it begins to proliferate uncontrollably and turns into a tissue that is stuck together without connection, which cannot exchange oxygen and waste with lymph and blood vessels. In other words, cell proliferation and tissue growth cause the central cells to be distanced from the blood vessel and consequently become oxygen-deprived. Therefore, they turn into dead cells, which over time, increase in number while being resistant to drugs and radiotherapy. Considering that radiotherapy and chemotherapy are highly dependent on the presence of oxygen in the tissue for reaction, what can be done for a dead cell without oxygen? In response, it must be said that oxygen must be injected into that cell with a targeted drug delivery method so that radiotherapy can respond better.
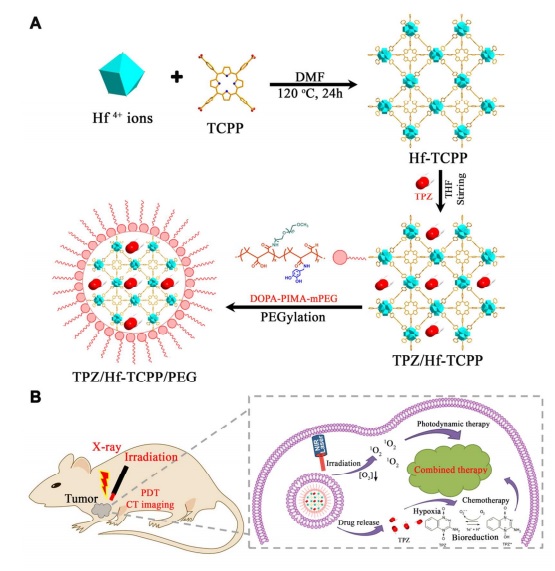
Oxygen can help destroy cancer cells in two ways: first, it can act as an oxidant as pure oxygen, or in the second pathway, light radiation with a specific frequency can create radical oxygens or single-electron hydroxyls that can cause the destruction of many structures, including DNA strands. In the research of this article, scientists reached a MOF (Metal-organic-framework) structure that can place a significant amount of the light-sensitive anti-cancer drug Tripazamine (TPZ), which has sufficient oxygen, inside this framework. This organic-metal structure, which includes zirconium metal and a porphyrin ligand molecule, showed potential capability in drug delivery. This drug carrier is rapidly degraded in the body and is also highly biodegradable, showing low toxicity. However, in this drug delivery, the drug must be spread very slowly and appropriately in the tissue and blood. Therefore, to achieve this feature, it must be covered with another biodegradable polymer called poly isobutylene-alt-maleic anhydride (PIMA). This coating showed potential capability in drug delivery and timing of drug release in the body of laboratory mice (the drug is held for a long time during release) and also this coating did not affect the crystalline structure of the framework.
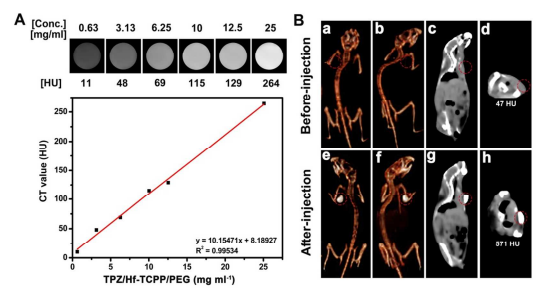
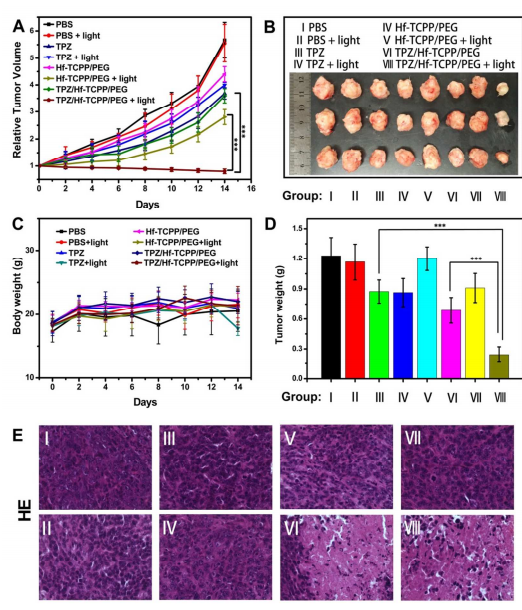
Without this coating, the drug has a half-life of 2.65 hours. In this article, an in-vitro system was used in conditions of 20% and 2% oxygen saturation, and good results were obtained for the organic-metal structure with polymer coating. In the in-vivo (intracellular and clinical) condition, these experiments were conducted on 40 laboratory mice with 8 possible pathways based on the presence or absence of several specific parameters: drug, light, organic-metal structure alone, etc., and in this case, the organic-metal drug with polymer coating TPZ/HF-TCPP/PEG showed very good results. Cell death and necrosis and apoptosis were identified by the Annexin diagnostic kit, and not so good results were identified for the anti-cancer drug TPZ and in the presence and absence of light alone for each. However, this apoptosis in the organic-metal structure of zirconium/tetrakis(4-carboxyphenyl)porphyrin (HF-TCPP) along with the anti-cancer drug Tripazamine (TPZ), which was placed inside it in a specific ratio, achieved the best result (increasing the concentration of the anti-cancer drug inside this structure gives the opposite result, so its concentration must be adjusted to a specific ratio!). In these experiments, devices such as X-ray diffraction variometers, ultraviolet radiation, Fourier-transform infrared conversion, biochemical cell death detection kit, scanning and transmission electron microscope, fluorescence, luminescence, and DLS were used.


klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)