Alternating Current Electrolysis for Organic Electrosynthesis: Trifluoromethylation of (Hetero)arenes
Posting date on solidfuturism: June 13th 2024
Published date: July 13th 2020
Authors: Sachini Rodrigo, Chanchamnan Um, Jason C. Mixdorf, Disni Gunasekera, Hien M. Nguyen,* and Long Luo
DOI: 10.1021/acs.orglett.0c01906
Abstract composer and editor: Seyed Amirhosein Mirsadri
In recent times, scientists have increasingly embraced electrochemical reactions for chemical synthesis. Electrosynthesis offers unique capabilities for chemical reactions, including easier control and identification of intermediate species or more selective reactions. Among the various electrosynthesis methods, those involving constant potential, constant current, flow systems, and membrane-based systems have been explored. However, paired synthesis methods, which have been less popular for some time, involve producing one substance at an electrode and converting it to another substance at a different electrode. This pathway is known as the “coupled pathway” because two direct reactions occur within the same cell, opposing each other.
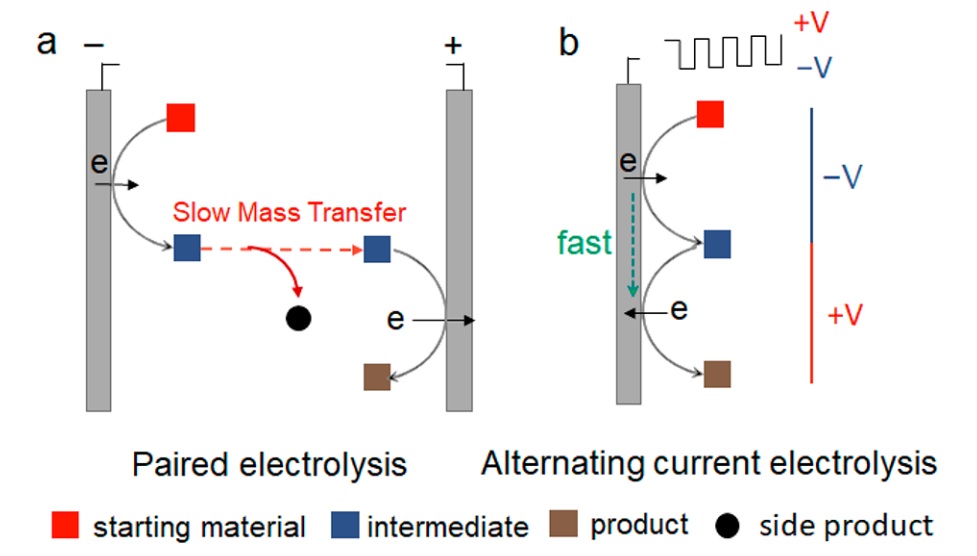
This method has both advantages and disadvantages. On the downside, it cannot support reactions with unstable intermediates that decay within milliseconds or longer time scales. To address this, scientists have attempted to control intermediate species and minimize side products using micrometer-scale electrolytes between the two electrodes. Although the cell design may seem complex, a recent study aimed to achieve the same coupled reactions that occurred between opposing electrodes, but this time on a single electrode surface. In other words, as soon as a substance is generated on the electrode surface, mass transfer no longer occurs, and the change in potential leads the same compound on the same electrode to transform into a new compound. Researchers discovered that the frequency and applied potential values play a crucial role.They investigated several different conditions, including direct current, alternating current, and various frequencies, as well as different applied potentials. For this purpose, they used a substance with an unstable radical resulting from a chemical reaction.
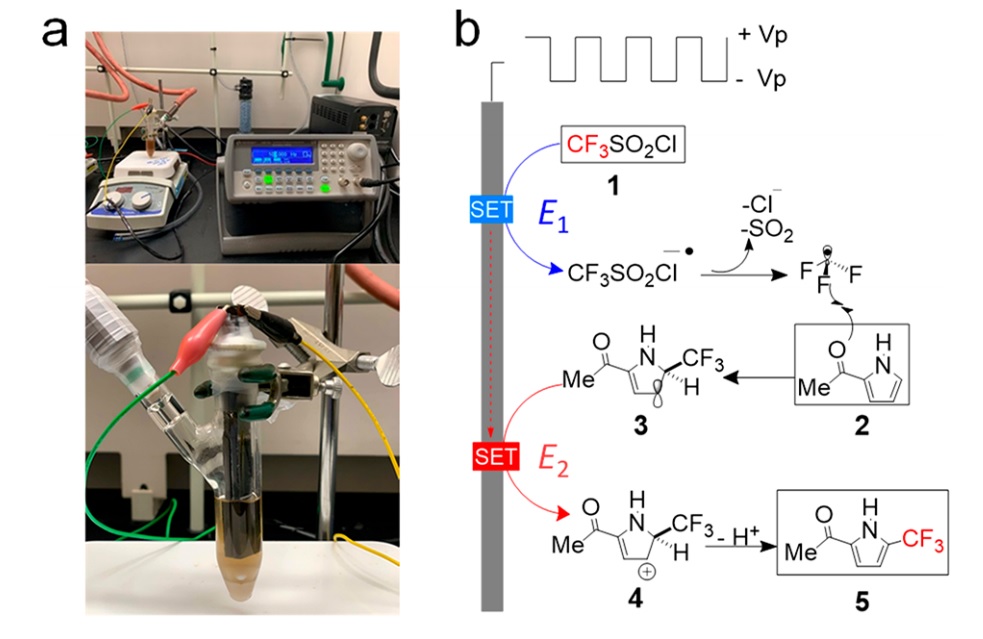
This method has both advantages and disadvantages. On the downside, it cannot support reactions with unstable intermediates that decay within milliseconds or longer time scales. To address this, scientists have attempted to control intermediate species and minimize side products using micrometer-scale electrolytes between the two electrodes. Although the cell design may seem complex, a recent study aimed to achieve the same coupled reactions that occurred between opposing electrodes, but this time on a single electrode surface. In other words, as soon as a substance is generated on the electrode surface, mass transfer no longer occurs, and the change in potential leads the same compound on the same electrode to transform into a new compound. Researchers discovered that the frequency and applied potential values play a crucial role.They investigated several different conditions, including direct current, alternating current, and various frequencies, as well as different applied potentials. For this purpose, they used a substance with an unstable radical resulting from a chemical reaction.
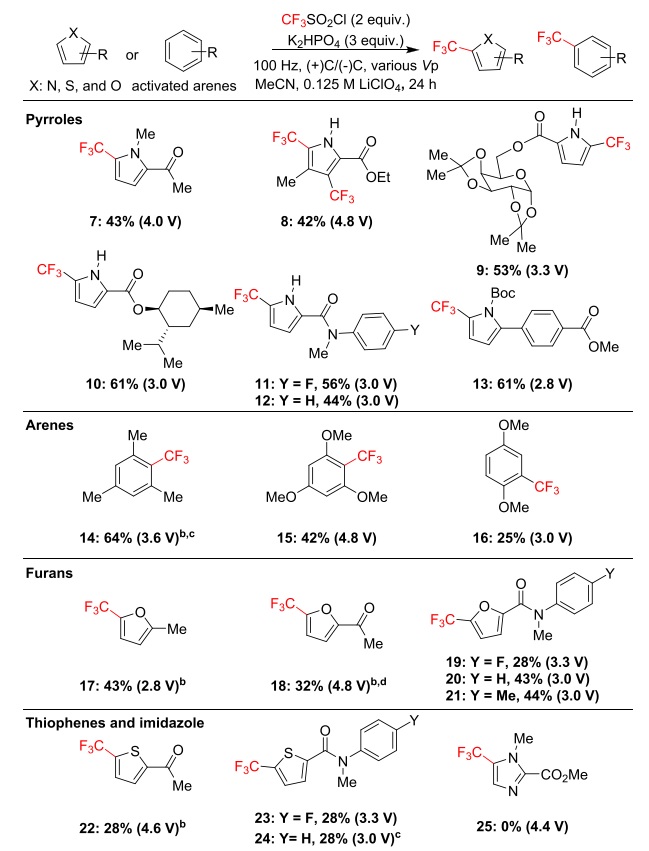


klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)