CATHODE MATERIAL IN THE ELECTROSYNTHESIS OF p-AMINOPHENOL FROM NITROBENZENEVOLTAMMETRIC APPROACH
Posting date on solidfuturism: May 9th 2024
Published date: April 1988
Authors: P SUBBIAH, M NOEL, S CHIDAMBARAM and K S UDUPA
URI: http://cecri.csircentral.net/id/eprint/1572
Abstract composer: Seyed Amirhosein Mirsadri
Nitrobenzene is one of the most important compounds in the chemical industry and serves as a precursor for many active substances. The reduction of nitrobenzene is one of the most well-known chemical reactions, which can lead to various active compounds. In this process, the nitro group on the benzene ring is ultimately converted to an amine, and the ring is eventually transformed into aniline.Researchers have attempted electrochemical reduction of nitrobenzene to produce the important compound para-aminophenol. However, this 4-electron pathway has encountered challenges. One of these challenges is the competitive reaction of the intermediate phenylhydroxylamine (C6H5NHOH) with para-aminophenol or aniline. Alongside para-aminophenol, aniline is also synthesized, which is not desired by those focusing on para-aminophenol synthesis. Researchers have made efforts to minimize this side reaction by optimizing temperature, current density, adding mineral salts, creating flow disturbances, and using alcohols with sulfuric acid (isopropanolic sulfuric acid).
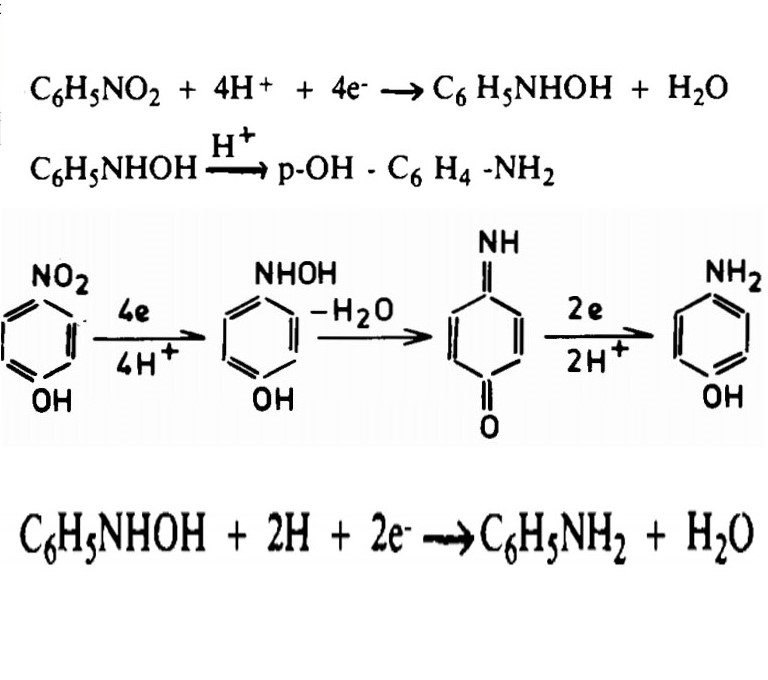
In this study, the synthesis of these products was investigated using different electrodes. Three electrodes were selected: lead (Pb), copper (Cu), and glassy carbon (GC). Sulfuric acid itself plays a crucial role in driving the reduction toward para-aminophenol. However, the lead electrode forms a sulfate film in the sulfuric acid environment, preventing the desired reaction. The copper electrode generates hydrogen gas at negative potentials (around -0.65 V), effectively producing hydrogen instead of reducing nitrobenzene. On the other hand, the carbon electrode has a more negative potential (around -1.25 V) and facilitates the reduction of nitrobenzene. However, the main challenge with the carbon electrode compared to copper is its lower charge transfer coefficient, which shifts the peak reduction potential toward more positive values, requiring higher energy input for the reduction.
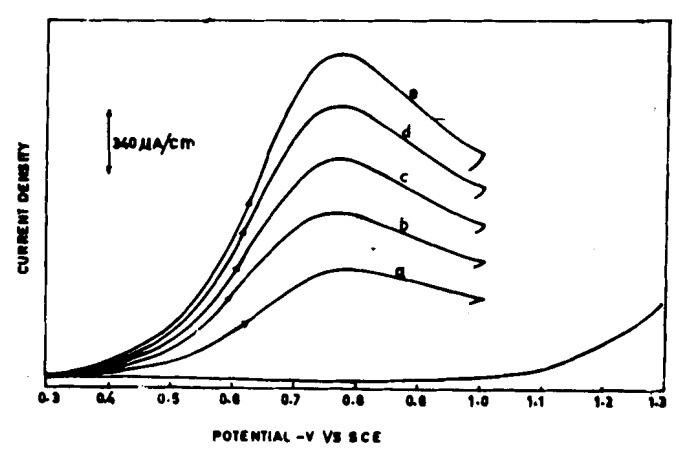
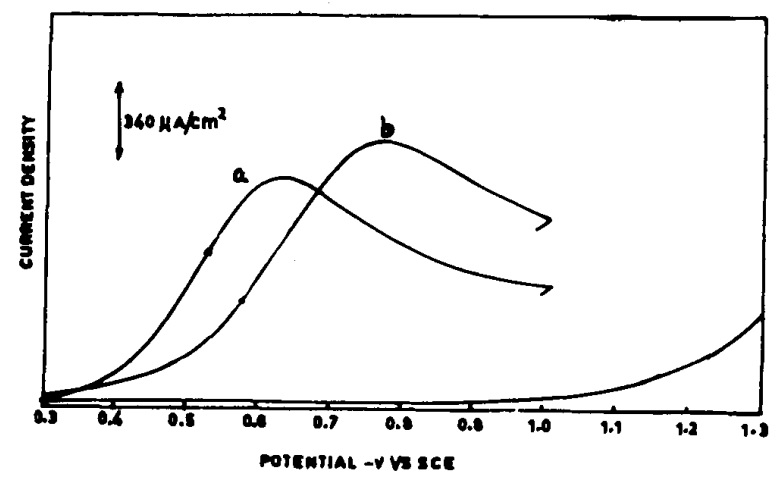
In experiments, molecules such as paranitrophenol and orthonitrophenol, derived from nitrobenzene, were successfully synthesized on the copper electrode via a 6-electron pathway. However, the difference between copper and glassy carbon lies in the proximity of the hydrogen reduction potential to the nitrobenzene reduction potential. Hydrogen production at the electrode surface can lead to aniline formation and reduce para-aminophenol yield. Therefore, for better efficiency, it is advisable to use the carbon electrode, which has a more negative reduction potential, to enhance para-aminophenol production. This study employed linear sweep voltammetry and investigated the effects of acid concentration on peak shift, as well as the impact of nitrobenzene and paranitrophenol concentrations and sweep rate on peak height. Ultimately, the glassy carbon electrode was selected as the best choice for reducing nitrobenzene to para-aminophenol.


klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)