Vitamin C-induced CO2 capture enables high-rate ethylene production in CO2 electroreduction
Posting date on solidfuturism: April 18th 2024
Published date: June 15 2024
Authors: Jongyoun Kim, Taemin Leeو Hyun Dong Jung, Minkyoung Kim, Jungsu Eo, Byeongjae Kang, Hyeonwoo Jung, Jaehyoung Park, Daewon Bae, Yujin Lee, Sojung Park, Wooyul Kim, Seoin Back, Youngu Lee & Dae-Hyun Nam
DOI: 10.1038/s41557-024-01495-z
Abstract composer: Seyed Amirhosein Mirsadri
As air pollution remains one of the main topics of discussion among scientists, the absorption and conversion of carbon dioxide are of significant importance. So far, many selective absorbers and catalysts have been introduced for this reaction. However, in a recent experiment, a group of scientists concluded that using ascorbic acid (vitamin C) can yield favorable results. If we consider the ascorbic acid present in fruit and imagine storing that fruit in a fruit warehouse, we can observe an atmosphere where carbon dioxide levels are consistently reduced. This is because ascorbic acid transforms into dehydroascorbic acid in the environment. Therefore, ascorbic acid can help retain carbon dioxide near the copper electrode. To achieve this, we need to provide a platform for ascorbic acid near the copper electrode using graphene quantum dots, which can form connections with carboxylic acid and hydroxide agents. Initially, graphene quantum dots are placed on the copper electrode, followed by coating them with ascorbic acid. The crucial aspect is understanding how this reaction proceeds.
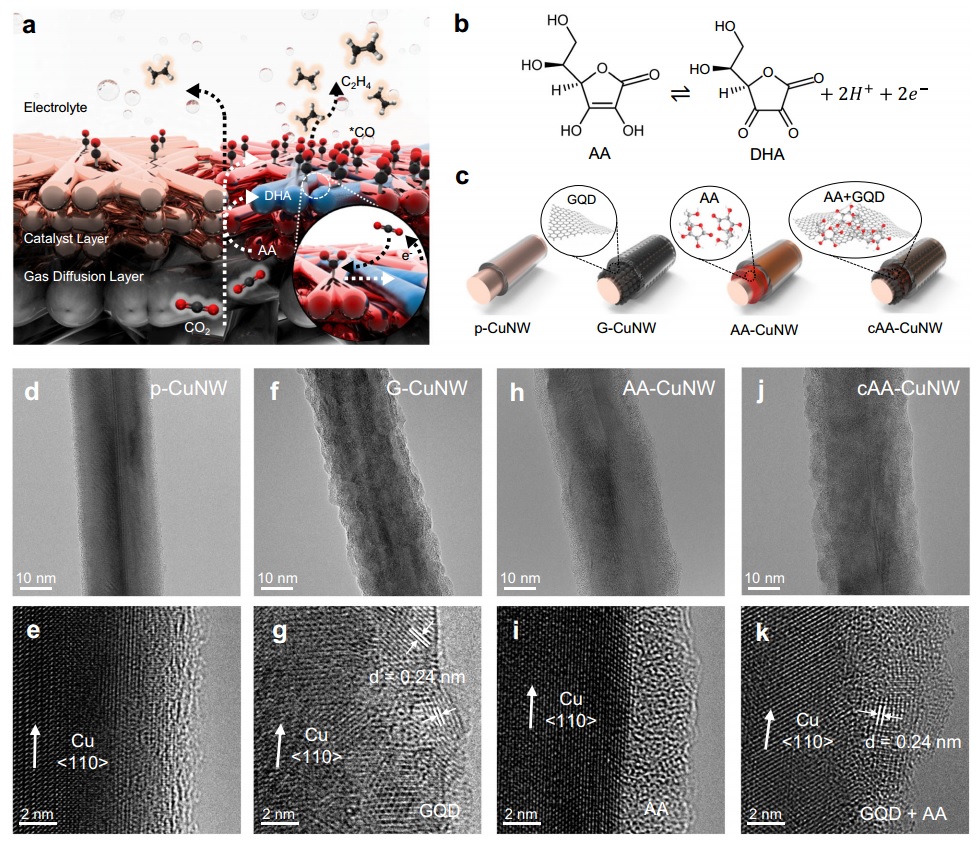
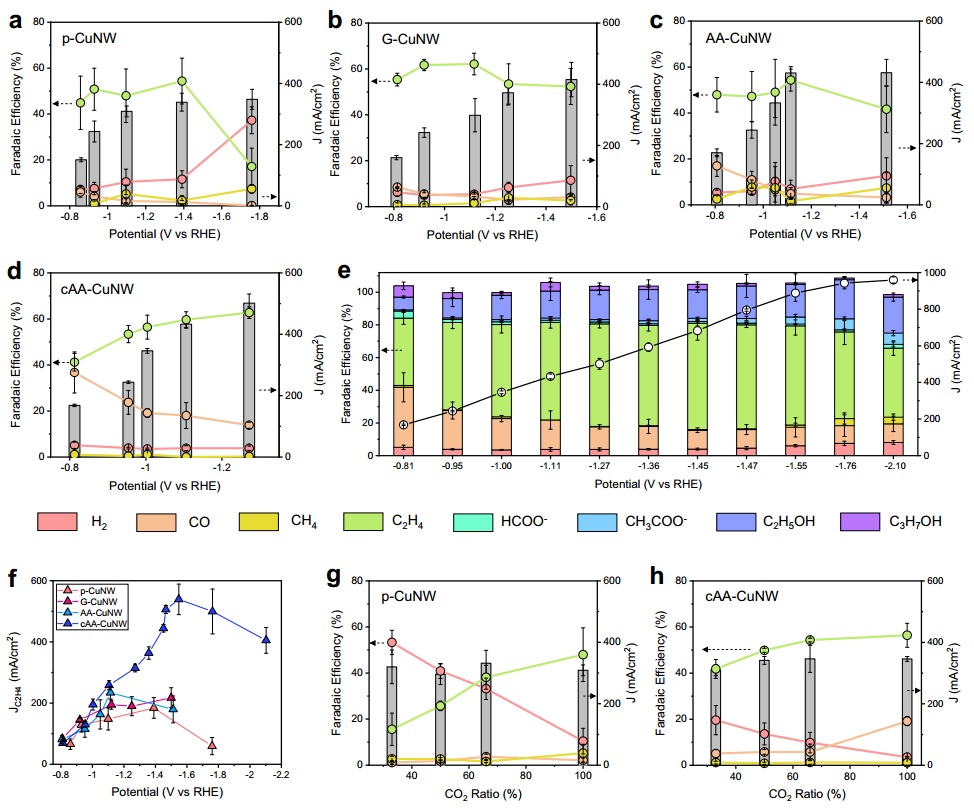
Essentially, we must create a carbonyl intermediate and then drive it toward dimerization. This dimerization pathway is not straightforward and requires catalytic selectivity to reach the ethylene compound. In this context, if we apply highly negative potentials to the bare copper electrode, the preference is for hydrogen gas production, resulting in higher hydrogen yields. However, in the presence of graphene quantum dots and ascorbic acid, this does not occur, and ethylene production increases (although its efficiency decreases). Thus, at low potentials, the pure copper electrode produced 45% ethylene and 6% carbonyl, whereas as the potential increased, the Faradaic efficiency decreased to 17%, and hydrogen production increased by 37%. An electrode containing graphene quantum dots but without ascorbic acid exhibited higher ethylene selectivity, reaching 62% efficiency at low potentials, while hydrogen accounted for only 10% at higher potentials. In the presence of ascorbic acid without graphene quantum dots, the carbonyl selectivity increased by 17% compared to the baseline.

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)

klsjuidhfasdgh.png)
klsjuidhfasdgh.png)
klsjuidhfasdgh.png)